Oxygen is one of the most essential elements on Earth, touching every aspect of our lives from the air we breathe to the water we drink. Yet, behind this seemingly simple element lies a fascinating atomic world. Understanding the number electrons in oxygen gives us insight not only into chemistry but into the very processes that sustain life. When we delve into the microscopic realm of atoms and subatomic particles, we begin to appreciate the delicate balance that allows oxygen to exist and function in countless ways. This article will take you on a journey through the unseen world of oxygen’s electrons, exploring the intricate details in a storytelling style that brings science to life.
Imagine a city at night. The lights flicker on as electricity flows through wires—without electrons, the city would be dark. Similarly, the electrons of oxygen light up the chemical world. These tiny particles, though invisible to the naked eye, dictate oxygen’s ability to bond with other elements, participate in combustion, form water, and support life at a cellular level.
Oxygen might seem ordinary because it is all around us, but the hidden world of its electrons tells a far more extraordinary story. The number electrons in oxygen—just eight in total—dictates how this element interacts with everything from the air we breathe to the water we drink and the energy our bodies produce. These tiny particles are responsible for chemical bonds, combustion, and even the processes that sustain life at a cellular level. By exploring these electrons, we begin to uncover the fascinating connections between atomic structure, chemistry, and the very essence of life itself.
Understanding Atoms and Electrons
Before diving directly into oxygen, it’s important to understand what atoms and electrons are. Everything in the universe is made up of atoms—tiny units that form matter. Each atom has a nucleus containing protons and neutrons, surrounded by electrons that orbit in shells or energy levels. These electrons are critical because they determine how an atom interacts with other atoms. In the case of oxygen, knowing the number electrons in oxygen is essential to understanding its chemical properties, such as why it forms water with hydrogen or why it’s highly reactive in combustion.
Electrons carry a negative charge and are incredibly tiny compared to the nucleus, yet their presence dictates an atom’s behavior in chemical reactions. The concept of electron configuration—the arrangement of electrons in an atom—helps scientists predict and explain chemical bonding, molecular shape, and reactivity. This microscopic dance of electrons is what makes chemistry not only fascinating but essential for life itself. Think of these shells as floors in a building. The first floor is fully occupied with 2 tenants (electrons), leaving the second floor with 6 tenants but space for 2 more. This creates a “desire” or chemical motivation for oxygen to seek two more electrons to complete its valence shell. This behavior explains why oxygen is reactive yet essential for stability in molecules like water (H₂O) and carbon dioxide (CO₂).
The Oxygen Atom: A Closer Look
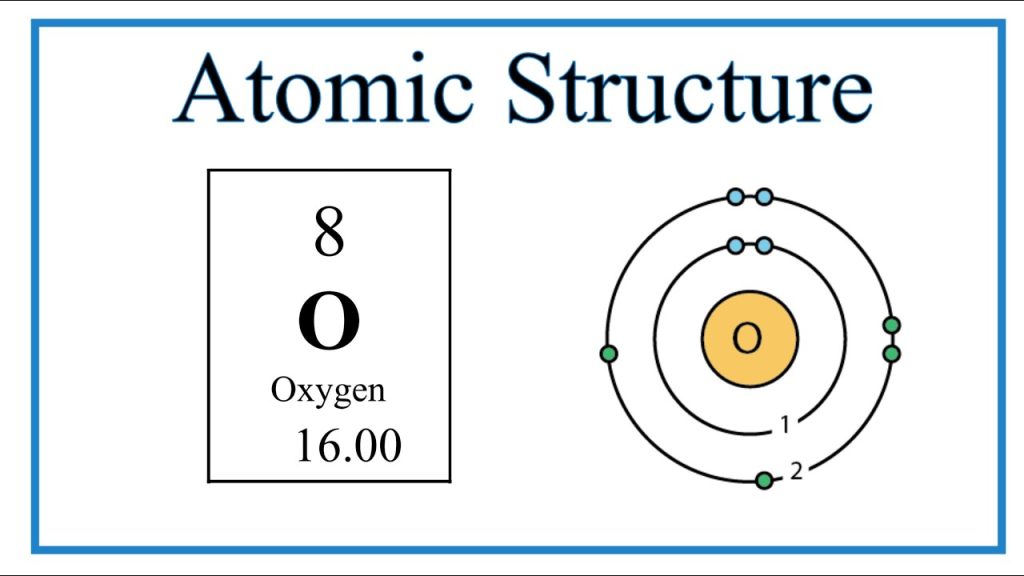
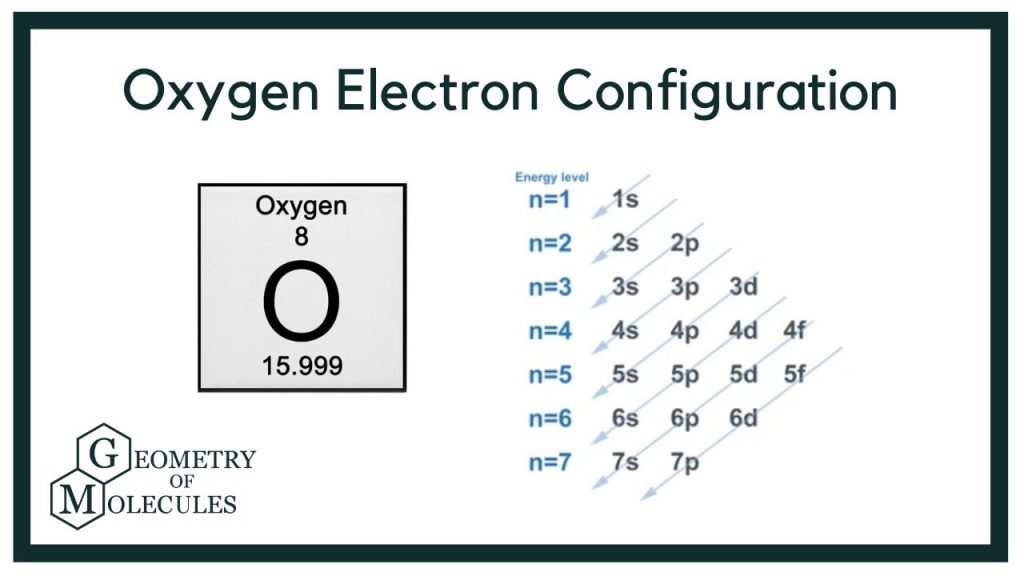
Oxygen is element number 8 on the periodic table. This number is not random; it indicates the number of protons in its nucleus, which also corresponds to the number electrons in oxygen when the atom is neutral. A neutral oxygen atom has 8 protons and 8 electrons. These electrons are arranged in shells, with 2 in the first shell and 6 in the second shell, giving oxygen a unique electron configuration of 1s² 2s² 2p⁴.
Think of oxygen as a social entity in the atomic world. It has a stable inner circle of 2 electrons (loyal friends) and a larger outer circle of 6 electrons (acquaintances) looking for partners to achieve harmony. This “desire” to bond explains oxygen’s electronegativity—the tendency to attract electrons from other atoms.
This configuration explains much about oxygen’s behavior. With six electrons in its outermost shell, oxygen is eager to gain or share two more electrons to achieve a stable octet, a state shared by noble gases. This desire drives oxygen’s chemical reactivity, particularly in forming molecules like O₂ and H₂O. Understanding the number electrons in oxygen allows chemists and biologists alike to predict how oxygen will behave in countless situations.
The Number Electrons in Oxygen: The Core Mystery
The number electrons in oxygen is fundamental to understanding why oxygen behaves the way it does. As mentioned, oxygen has 8 electrons, with 2 in its inner shell and 6 in the outer shell. This outer shell, called the valence shell, is crucial because it is where chemical reactions occur. The tendency of oxygen to form two bonds in most compounds stems directly from the arrangement of these 6 valence electrons.
Oxygen’s electrons also give it unique properties in forming radicals and reactive oxygen species (ROS), crucial for biological signaling. Even small changes in electron arrangement can alter reactivity dramatically, highlighting the significance of understanding the number electrons in oxygen.
Imagine a busy city where houses (electrons) are tightly packed in a neighborhood (the atom). Oxygen’s outer shell has 6 “houses” and craves 2 more to make a complete “block,” leading it to bond with other elements. This simple fact underlies everything from water formation to the respiration process in our cells. Knowing the number electrons in oxygen is not just an academic exercise—it reveals the very blueprint of life.
How Electrons Define Oxygen’s Chemical Behavior
The chemical behavior of oxygen is dictated by its electrons. Oxygen is highly electronegative, meaning it strongly attracts electrons in a bond. This property allows it to pull electrons towards itself in compounds, creating partial charges and enabling hydrogen bonding—a phenomenon critical for water’s unique properties.
Imagine oxygen as a skilled dancer. Its 6 electrons in the outer shell determine its dance moves—how it spins, twirls, and partners with other atoms. Without understanding the number electrons in oxygen, we would never comprehend why water forms at room temperature or why oxygen reacts differently with metals compared to nonmetals.
For instance, in water (H₂O), oxygen forms two covalent bonds with hydrogen atoms. Its 6 valence electrons share with hydrogen’s single electrons to form stable pairs. This electron sharing is why water is liquid at room temperature, why it dissolves so many substances, and why it’s vital for all life forms. The number electrons in oxygen is at the center of these interactions, shaping the world as we know it.
Oxygen in Everyday Life: More Than Just Air
We often take oxygen for granted. Yet, its electrons are at the heart of many everyday processes. From rust forming on metal to the electricity generated in batteries, oxygen’s 8 electrons play a silent but powerful role. For example, when iron rusts, oxygen atoms interact with iron electrons in a redox reaction, forming iron oxide—a process that shows how reactive oxygen can be.
Even in water purification, oxygen’s electrons enable oxidation reactions that remove harmful pollutants, demonstrating that these 8 electrons are silently sustaining life, industry, and the environment.
Similarly, oxygen is used in medical therapies, metal cutting, and water purification. In every application, its behavior can be traced back to the number electrons in oxygen and their desire to achieve stability. Understanding these electrons allows engineers and scientists to harness oxygen safely and effectively.
The Role of Oxygen in Biological Systems
Oxygen is the lifeblood of biological systems. Every living organism—from the tiniest bacteria to humans—depends on oxygen to survive. The number electrons in oxygen determines how it interacts with other molecules, especially in respiration, where oxygen acts as the final electron acceptor in the electron transport chain.
Picture your body as a bustling city at night. The mitochondria are the power plants, generating energy for every activity. Oxygen swoops in, accepting electrons in a highly controlled process that produces ATP, the energy currency of life. Without the precise arrangement of its 8 electrons, oxygen could not accept electrons effectively, and life as we know it would cease.
Imagine mitochondria as factories producing electricity. Oxygen’s electrons are the crucial component in this power generation, making them the hidden heroes of cellular energy. Without these electrons, our muscles would fail, brains would stop thinking, and life would halt.
Oxygen also plays a role in detoxification, immune responses, and maintaining cellular balance. Its electrons are central in forming reactive oxygen species (ROS), which, in controlled amounts, signal cells to repair damage. Too many ROS, however, can cause oxidative stress, demonstrating the delicate balance dictated by oxygen’s electron configuration. Understanding the number electrons in oxygen is essential to appreciating these complex biological processes.
Oxygen and Combustion: Fire and Life
The reactive nature of oxygen makes it vital for combustion. When wood burns in a fireplace or fuel powers an engine, oxygen’s electrons are actively involved in breaking and forming chemical bonds. In combustion, oxygen molecules collide with fuel, accepting electrons and releasing energy in the form of heat and light.
Think of oxygen as an enthusiastic partner in a dynamic dance. Its electrons seek to pair with fuel molecules, releasing energy in spectacular ways. The precise behavior of these electrons explains why some materials burn faster, slower, or not at all. Without the number electrons in oxygen, controlled combustion would be impossible, and the modern world of energy would be vastly different.
Think of oxygen as a partner in a fiery dance. Its electrons eagerly pair with those from fuel molecules, creating a spectacular reaction. Without the number electrons in oxygen being exactly 8, these reactions could not occur efficiently, and combustion would be unpredictable. This property is why oxygen is both a giver of life—through energy production—and a potential destroyer when uncontrolled fires occur.
This dual nature—supporting life yet enabling destruction—underscores the critical importance of oxygen’s electrons in the physical world. Understanding these electrons allows scientists to harness energy safely, from industrial processes to powering our homes.
Advances in Science: Oxygen and Modern Technology
Oxygen’s role extends beyond life and fire; it is integral to modern technology. In medicine, oxygen therapy saves lives, while in engineering, oxygen is used in welding, cutting, and even rocketry. The secret to these applications lies in the number electrons in oxygen and how these electrons participate in chemical reactions.
In electronics, oxygen’s electrons influence the behavior of semiconductors, affecting conductivity and stability. In environmental science, oxygen plays a role in water treatment, helping to break down pollutants through oxidation. Advances in nanotechnology and materials science continue to reveal ways oxygen’s electrons can be manipulated to create innovative solutions.
Imagine scientists as sculptors, shaping the world at a molecular level. Oxygen is their clay, and its electrons are the tools that allow them to mold matter in precise ways. Without understanding the number electrons in oxygen, these technological breakthroughs would not be possible.
Scientists are architects of the unseen, and oxygen’s electrons are their building blocks. By understanding the number electrons in oxygen, they can design solutions for energy, health, and environmental sustainability.
Surprising Facts About the Number Electrons in Oxygen
Even after centuries of study, oxygen never ceases to surprise us. Here are some fascinating facts tied to the number electrons in oxygen:
- Oxygen can form multiple bonds: Its six valence electrons allow it to share electrons in double bonds, as seen in O₂, or triple bonds in rare compounds like N₂O₃.
- Oxygen in the universe: Oxygen is the third most abundant element in the universe, and its electron configuration influences stellar chemistry.
- Oxygen isotopes: The number of neutrons may vary, but the electron count remains the same in neutral atoms, maintaining chemical behavior.
- Electronics and oxygen: Its electrons are central to oxygen sensors and fuel cells.
- Reactive but balanced: Oxygen’s 8 electrons create a delicate balance of reactivity and stability, vital for both life and industrial processes.
Together, these fascinating facts show just how important the number electrons in oxygen is in shaping both the microscopic and macroscopic world. From forming life-sustaining molecules like water to creating protective ozone in the atmosphere, oxygen’s electrons play a critical role in chemistry, biology, and the environment. Understanding these electrons not only deepens our appreciation of oxygen’s versatility but also highlights the intricate connections between atomic behavior and the world around us.
Each of these facts demonstrates that understanding the number electrons in oxygen is not just academic—it’s crucial for science, industry, and health.
Misconceptions About Oxygen and Electrons
Despite its familiarity, oxygen is often misunderstood. A common misconception is that oxygen is inert. In reality, its electrons make it highly reactive, especially when forming compounds with metals or in biological processes. Another myth is that oxygen is always harmful as a reactive oxygen species. Controlled ROS production is essential for cellular signaling and repair.
A common misconception is that oxygen is always dangerous as a reactive oxygen species (ROS). In reality, ROS produced by oxygen’s electrons play important roles in signaling, healing, and regulating cellular functions. It’s the balance of these electrons, rather than their mere presence, that determines whether oxygen is beneficial or harmful.
Misunderstandings often arise from oversimplified textbooks or popular science articles. By focusing on the number electrons in oxygen, we can clarify why oxygen behaves as it does in different environments. These misconceptions, when corrected, open doors to better scientific literacy and safer applications in technology and medicine.
The Future of Oxygen Research
Research into oxygen is far from over. Scientists are exploring new ways to manipulate oxygen’s electrons for energy, medicine, and materials science. Innovations in artificial photosynthesis aim to use oxygen’s reactivity to generate sustainable energy. In medicine, understanding electron behavior in oxygen could lead to better treatments for oxidative stress-related diseases.
The study of oxygen in extreme environments, from deep-sea vents to outer space, also depends on knowing the number electrons in oxygen. How electrons behave under pressure, radiation, or in rare compounds could revolutionize chemistry, biology, and physics. The future promises discoveries that could change how humanity interacts with this fundamental element.
As we look to the future, the number electrons in oxygen will continue to guide groundbreaking research in energy, medicine, and environmental science. Scientists are exploring ways to manipulate these electrons to create cleaner fuels, more efficient batteries, and advanced therapies for diseases caused by oxidative stress. Even in space exploration, understanding oxygen’s electrons could help sustain life on other planets or develop new propulsion technologies. Each discovery reveals just how much these 8 tiny electrons influence both our daily lives and the broader frontiers of science.
Visit our website for more updates and stories
Conclusion: Why Knowing Oxygen’s Electrons Matters
The number electrons in oxygen is more than just a scientific fact—it is the foundation of life and the driving force behind countless chemical reactions. Oxygen’s 8 electrons shape everything from water formation to the energy cycles that power our bodies and support life on Earth.
These electrons also guide innovations in science, medicine, and technology, showing how understanding oxygen allows us to harness its potential safely and effectively. Appreciating the number electrons in oxygen helps us see the invisible forces shaping chemical reactions, powering our bodies, and influencing the environment.
The story of oxygen’s electrons reminds us that even the smallest particles can have immense power. By exploring these electrons, we gain both knowledge and wonder at the hidden forces shaping our world—a story that continues to unfold with every discovery.