When I first stepped into the chemistry lab as a student, I didn’t expect that one of the most memorable lessons would be about something as seemingly simple as the molecular mass of acetic acid. The glass beakers sparkled under the bright lights, the scent of various chemicals filled the air, and in front of me sat a bottle labeled CH₃COOH. It was vinegar in its purest form, yet the numbers behind it told a deeper story.
That day, our professor smiled and said, “Sometimes the smallest molecules have the biggest impact.” As we explored its composition, I realized that the molecular mass of acetic acid wasn’t just a number — it was the key to understanding why this compound behaves the way it does in science and in everyday life. It made me curious about how atomic masses combine to form molecular masses, and why those numbers are so important in chemical reactions.
Understanding the Molecular Mass of Acetic Acid
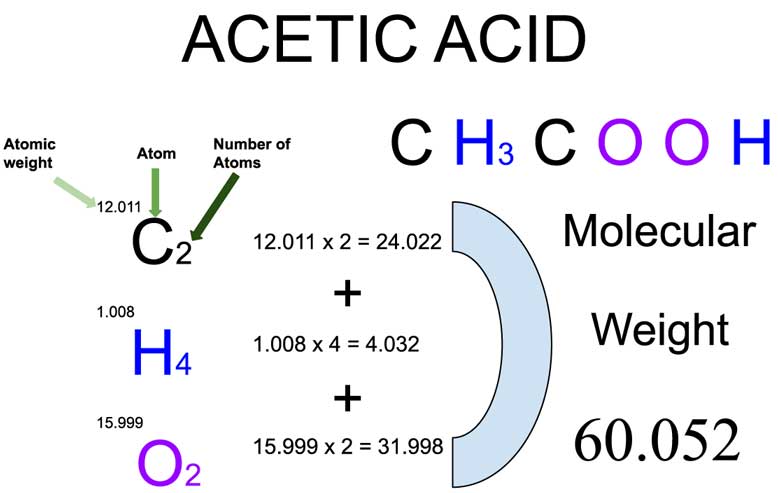
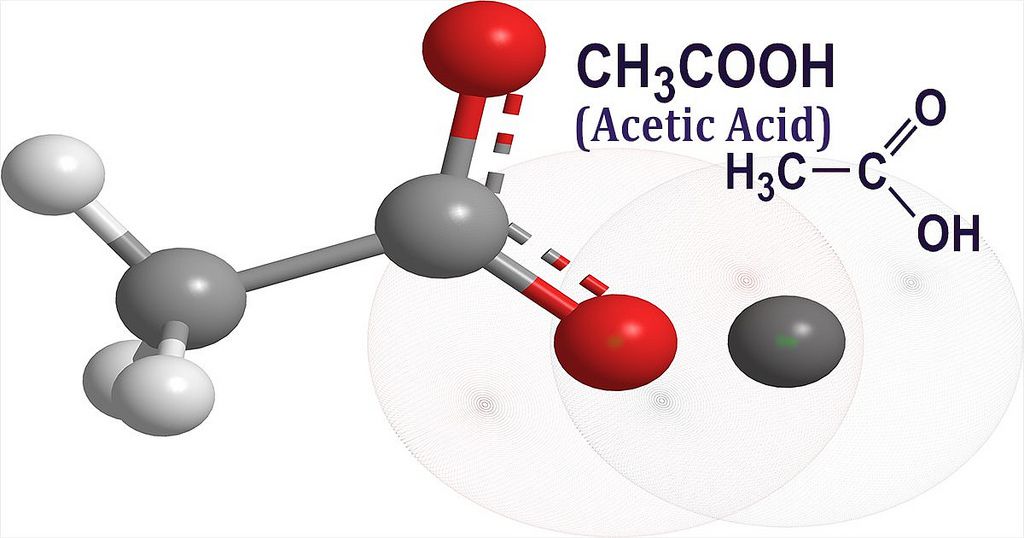
The molecular mass of acetic acid comes from adding up the atomic masses of all the atoms in its formula: CH₃COOH. Each carbon, hydrogen, and oxygen atom contributes to the total.
Here’s how it works:
- Carbon (C) has an atomic mass of about 12.01, and there are two carbon atoms.
- Hydrogen (H) has an atomic mass of about 1.008, and there are four hydrogen atoms.
- Oxygen (O) has an atomic mass of about 16.00, and there are two oxygen atoms.
When you add them up:
(2 × 12.01) + (4 × 1.008) + (2 × 16.00) = 60.05 g/mol
This number — 60.05 grams per mole — might look ordinary, but it is vital in chemistry because it helps determine how much of the substance is used in reactions and solutions.
The Story of Acetic Acid Through Time
Acetic acid’s history is as rich as its chemical properties. Thousands of years ago, ancient civilizations didn’t know the term “molecular mass,” but they used vinegar, which is a diluted form of acetic acid, for cooking, cleaning, and preserving food.
In ancient Egypt, vinegar was even part of medicinal remedies. During the Roman era, soldiers carried vinegar to mix with water, creating a refreshing and safe drink. Over time, scientists began to isolate and study the pure form of acetic acid, leading to a deeper understanding of its structure and molecular mass.
Today, it remains one of the most studied and widely used acids, proving that small molecules can stand the test of time. Its journey from ancient kitchens to high-tech laboratories is a testament to its versatility.
How Scientists Measure Molecular Mass
Determining the molecular mass of acetic acid isn’t guesswork. In modern labs, scientists use precise instruments like mass spectrometers. These machines measure the mass-to-charge ratio of ions, giving exact molecular weights.
In the classroom, however, students usually rely on the periodic table and simple calculations. By adding up the atomic masses from the table, they can easily find the molecular mass. This process helps develop problem-solving skills and builds a foundation for more complex chemical studies.
Interestingly, the same calculation method works for countless other compounds, from simple gases to complex proteins. Understanding this one calculation opens the door to a much broader world of chemistry.
Real-Life Uses of Acetic Acid
Once you know the molecular mass of acetic acid, you can apply that knowledge in many practical situations. Acetic acid is more than a chemistry example — it’s a versatile compound used in multiple industries.
- Food Industry: In vinegar, it adds flavor and preserves freshness.
- Medical Field: Used in diluted form to treat certain infections.
- Industrial Manufacturing: Essential for producing synthetic fibers, plastics, and dyes.
- Laboratories: Used as a reagent in various chemical reactions.
Each of these applications relies on precise measurements, and understanding the molecular mass is part of that accuracy. Imagine a factory making a batch of vinegar; knowing the exact molecular mass ensures consistent quality. Even in textile production, where acetic acid is used in dyeing processes, small measurement errors can lead to big differences in results.
Why Knowing the Molecular Mass of Acetic Acid Matters
In science, details matter. The molecular mass of acetic acid is more than a trivia fact — it’s a cornerstone for experiments, manufacturing, and quality control. Without knowing it, chemists couldn’t accurately prepare solutions, manufacturers couldn’t ensure consistent products, and educators couldn’t teach chemical principles with precision.
It’s also a great example for students learning about chemical formulas and the periodic table. By studying this molecule, they see how a simple calculation connects theory to real-world applications.
Even in our kitchens, when vinegar helps keep pickles fresh, the underlying chemistry is guided by the same principles that a scientist uses in a laboratory. From salad dressings to synthetic fabrics, the story of acetic acid shows that chemistry is everywhere.
Visit our website for more updates and stories
Final Thoughts on This Small but Mighty Molecule
The day I learned about it in the chemistry lab was the day I realized that science often hides its most fascinating stories in the smallest of details. That single value, 60.05 g/mol, connects ancient vinegar makers with modern scientists, making the molecular mass of acetic acid a timeless piece of knowledge. And perhaps, the next time you see a bottle of vinegar, you’ll think not just about its taste — but about the fascinating chemistry that makes it possible.